| II Content of licensing:Overseas IVD registrationIV Fees:No chargeVI List of application documents:Data number (1) Overseas IVD registration application form;Data number (2) |
I. Project name: initial registration of imported medical devices in China
II. Content of licensing: Overseas IVD registration
III. Basis for implementation: "Regulations for Supervision and Administration of Medical Devices ", "Provisions for Medical Device Registration", "Provisions for Registration of IVD (Interim)"
IV. Fees: No charge
V. Quantity restrictions: No quantity limit for the licensing
VI. List of application documents:
Data number (1) Overseas IVD registration application form;
Data number (2) Certification of overseas manufacturers;
Data number (3) Certifying documents of foreign government authorities�� approval for the product to be marketed in the country (region) of origin;
Data number (4) Certifying documents of overseas manufacturer��s compliance of "GMP" in countries or regions of origin, or other QMS certification documents;
Data number (5) Copies of designated agent��s letter of entrustment /commitment, business license or institutional registration certificate (photocopy);
Data number (6) Copies of letter of entrustment /commitment of designated registration agent, business license or institutional registration certificate (photocopy);
Data number (7) A statement of authenticity of the documents submitted;
Data number (8) Summary data
Data number (9) Package insert
Data number (10) Intended product standards and its preparation explanations;
Data number (11) Registration testing report;
Data number (12) Research data for major raw materials;
Data number (13) The research data for main production technology and reaction system;
Data number (14) Analysis of performance evaluation data;
Data number (15) Determination of reference value (reference range);
Data number (16) Stability research data;
Data number (17) Clinical trials data
Data number (18) Production and self-test records;
Data number (19) Sample of packaging and labeling;
Data number (20) Quality management system evaluation report.
VII. Requirements for application dossier:
(I) General requirements for the dossier:
1. The dossier shall be bound into books.
2. The first page of the dossier is a list of application documents, which shall be arranged in order as per ��General requirements��. Each document shall be separated by labeled slip sheets and marked with data item number.
3. The dossier shall be in one full copy (product standards and product package inserts shall be in duplicate).
4. All documents in the dossier compiled by the applicant or agent shall be printed in A4 size paper, the contents shall be complete, clear, without alteration. Documents issued by the Government and other agencies shall be provided at the original size, where the bound volumes shall not be split without authorization.
5. The copies of dossier shall be clear and consistent with the originals.
6. The contents of application document (application form, approvals for marketing, product standards, testing reports, and Package inserts) shall be consistent. The trade name and English name, if any, shall be marked.
7. The application documents shall be in Chinese, and translated documents shall be accompanied with the originals.
8. Product name shall meet the nomenclature rules stipulated by the "Provisions for Registration of IVD (Interim)".
9. The electronic versions of the following registration document shall also be submitted:
(1) Application form
(2) Abstracts of the literature review, wherein: the intended use of the product (500words or less), product description (200 words or less), biological safety explanations (100 words or less),summarizes and evaluation of the main findings (200 words or less) and others (200 words or less);
(3) Intended product standards and its preparation explanations;
(4) Package inserts
The above electronic documents, except for the application forms, shall be WORD document without exception, which can be edited and modified.
(II) Specific requirements for application documents:
1. Overseas IVD registration application form;
(1) The ��electronic application software 2010 for medical devices (IVD) registration�� can be downloaded from www.cfda.gov.cn;
(2) All entries of the form shall be filled in accordance with the requirements described in the instructions;
(3) The ��applicant�� item shall be filled in English;
(4) For remission or exemption of clinical trials for rare disease, special diseases and other conditions, the applicant shall submit such an application along with the registration application documents, stating the reasons therein and provide relevant documentation.
2. Certification of overseas manufacturers;
(1) Qualifications for legal production of medical devices, if the documents have product category description, the category shall cover the applied product;
(2) The name of the manufacturer shall be consistent with that in the application form;
(3) Copies shall be signed and sealed by the original certificate issuers or notarized by the local notary organizations;
(4) All the above certification shall be All the above certification shall be within the validity period.
3. Overseas governmental authorities�� approval documents for marketing of the product in the country (region) refer to the documents issued by the government authorities of the country (region) of the applicant
(1) If the marketing-approval documents are copies, they shall be sealed by the original authorities who issue the documents, or notarized (completely) by corresponding notary organizations.
(2) For overseas products not subject to medical device market approvals, the applicant shall provide:
�� Documents proving that the products are not managed as medical devices in registration;
�� Documents proving that the products are legally marketed in the country of origin.
(3) Certifying Documents (if any) for approvals of change issued by the country (region) of origin.
(4) All the above certification shall be within the validity period (if any).
(5) The name of the product and packaging specifications (if any) in the Documents shall be consistent with the applied product.
(6) The name of the manufacturer shall be consistent with that in the application form;
(7) If the manufacturer name is inconsistent with the name of the applicant in the registration application form, such as in mergers, acquisitions, and other cases, the applicant must provide the corresponding certifying documents.
4. Certifying documents of overseas manufacturer��s compliance of "GMP" in countries or regions of origin, or other QMS certification documents;
(1) Copies shall be signed and sealed by the original certificate issuers or notarized by the local notary organizations;
(2) All the above certification shall be within the validity period (if any).
(3) QMS documentation shall include the applied product;
(4) The name of the manufacturer shall be consistent with that in the application form;
(5) If the manufacturer name is inconsistent with the name of the applicant in the registration application form, such as in mergers, acquisitions, and other cases, the applicant must provide the corresponding certifying documents.
5. Copies of designated agent��s letter of entrustment /commitment, business license or institutional registration certificate (photocopy);
(1) Designated agent��s certificate of entrustment;
(2) Certificate of the applicant��s registered office in China or copy of the business license of its domestic agency (photocopy);
(3) Agent��s certificate of entrustment.
6. Copies of letter of entrustment /commitment of designated registration agent, business license or institutional registration certificate (photocopy);
(1) Copies of letter of entrustment��
(2) Certificate of the applicant��s registered office in China or copy of the business license of its domestic agency (photocopy);
(3) Registration agency��s letter of commitment.
7. A statement of authenticity of the documents submitted;
(1) The Statement shall the list of all application documents;
(2) Original statement shall be issued by the applicant;
(3) Chinese statement shall be issued by an agent;
(4) The Statement shall express commitment of legal responsibility.
8. Package insert
(1) Product��s original package inserts and complete translation;
(2) The format of the package inserts used in China shall be in line with the "Guidelines for Compiling IVD Package Inserts";
(3) If the original packages have no inserts in original language, the applicant shall provide explanatory documents and compile Chinese package inserts for products used in China in accordance with the "Guidelines for Compiling IVD Package Inserts";
(4) The package inserts for products used in China shall be in duplicate, and attached with a statement of consistency of the two.
9. Intended product standards and its preparation explanations;
(1) The intended product standards and preparation explanations shall include both English and Chinese version, the Chinese version shall be in duplicate, and attached with a statement of consistency of the two;
(2) The Chinese version of the intended product standards shall be in line with the requirements of GB/T1.1;
(3) The English version of the intended product standards shall be signed by the applicant, the Chinese version shall be signed by the applicant or its agent;
(4) If the product adopts national standards and industry standards as applicable standards, the applicant shall submit:
�� A statement of the applied product��s compliance with national standards and industry standards;
�� A statement of commitment for post-marketed product quality;
�� And explanations of the classification of product packaging specifications.
10. Registration testing report;
(1) Originals of registration testing reports issued by competent testing institutions accredited by CFDA;
(2) The packaging specifications of the testing product shall be within the range of the registration application;
(3) The testing type shall be import registration testing;
(4) For Class III products, registration testing of three consecutive production sample batches shall be conducted.
11. Clinical trials data
(1) Overseas clinical trial data
(2) Specific requirements for domestic clinical trial data:
�� Class III products: the applicant shall carry out clinical trials in no less than three (including three) provincial health care institutions.
�� Class II products: the applicant shall carry out clinical trials in no less than two (including two) provincial health care institutions.
�� For special-purpose products, the applicant can conduct clinical trials in qualified municipal-level and above centers for disease control, specialized hospitals or inspection and quarantine institutions, detoxification center and other institutions.
�� Clinical trial agreement: signed by clinical trial institutions and the agent or the applicant.
�� Clinical trial protocol: signed and sealed by the primary leaders (signature), clinical trial organizations (seal), person and institution in charge of statistics, the applicant, and the ethics committee (leading unit).
If the clinical trials aren��t subject to the consent of the Ethics Committee, the leading unit shall still provide a description of the ethical issues with signature.
�� Clinical trial reports issued by the institutions, the cover of which shall include:
a. Product name used in the clinical trial;
b. Clinical trials start and finish dates;
c. Signatures of all main person responsible for the clinical trials, the signature of clinical trials institution, the signature and seal of Statistics head and the stamp of the applicant;
d. The contact and contact information of the product registration applicant, the reporting date, the locations for archiving of original data.
�� Summary report of the results of all clinical trials:
a. The reports shall be completed by the leading unit or the applicant;
b. the contents of the cover shall be the same as those of the clinical trial reports of various clinical trial institutions.
�� Detailed information of clinical trials, including the results of all clinical trials, other testing methods or other basic information of diagnostic reagent products, such as testing methods, sources of diagnostic reagent products, package inserts and registration approvals and so on.
�� The batch numbers of clinical trial samples must match with those used by the applicant for intended product standards testing, and the pre-clinical testing reports can be either the applicant��s self-test report, or testing report issued by other competent testing institutions entrusted by the applicant.
�� For calibration products, quality control products, and reference solution etc., clinical trial data need not to be provided.
�� The ��seals�� of clinical trial institutions mentioned in this section refers to the official seals of clinical trial institutions and/or competent departments responsible for the trials in clinical trial institutions.
12. Production and self-test records;
Provide copies of three consecutive batches production and self-test records.
13. Quality management system evaluation report (if any).
Overseas QMS assessment report completed within valid registration period and issued by CFDA. For Class I products, the manufacturer��s QMS self test report shall be provided if necessary.
14. Dossier No.8, 12, 13, 14, 15, 16 and 18 shall be completed overseas by the applicant.
15. Application dossier shall be original, unless otherwise noted, and signed by the applicant, the Chinese version shall be signed and sealed by the agent. "signature and seal" of the dossier in original language: signature of the applicant's legal representative, responsible person, or signature plus stamp of the organization, notarized document issued by notary institution in jurisdiction of the applicant; "signature and seal" of the dossier in Chinese: Agents�� organization seals, or seals of their legal representative, responsible person and organizations.
VIII. Application process diagram:
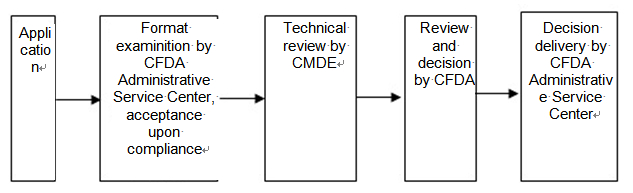
IX. Approval procedures:
(I) Acceptance:
After the application is filed to the Administrative Acceptance Service Center, and the application dossier submitted is in accordance with the list in VI of this Notice, the format examination shall be conducted by the work staff of the Center in accordance with the "Notice on the issuance of the formats and requirements for IVD registration application dossier" (SFDA Department of medical device supervision [2007] No.609). Where the application item is not subject to administrative approval according to law, a decision of non-acceptance shall be notified to the applicant immediately. Where the application item is not within the functions of the administrative authority, a decision of non-acceptance shall be made immediately, and the applicant shall be informed to launch the application to relevant administrative authority. Where in the application dossier, there are errors that can be corrected on the spot, the applicant shall be allowed to make the corrections on the spot. Where the application dossier is incomplete or does not meet the statutory format, the applicant shall be informed of all the supplements and amendments to be made once and for all on the spot or within five days. If the applicant is not informed within the above-mentioned period, the application shall be deemed as accepted from date of receipt of application materials. Where the application dossier is within the functions of the administrative authority, the application materials are complete and comply with the statutory format, or where the applicant has submitted all supplements and amendments in accordance with the requirements of the administrative authority, the application for administrative approval shall be accepted.
(II) Review:
Once the application is accepted, Administrative Service Center shall deliver the application dossier to the CMDE for technical review, which includes product testing and expert review, and not exceed 60days. In case of any rectification is required after the expert review, the rectification time shall not be included in the time frame for licensing.
(III) Licensing decisions:
Upon receipt of the dossier after CMDE technical review, CFDA shall make a decision for registration within 30 days, and written explanations shall be made for rejection of registration.
(IV) Delivery:
Within 10 days from the date of decision making of administrative approval, CFDA Administrative Service Center shall deliver the decision to the applicants.
X. Commitment time frame:
The administrative licensing decision shall be made within 90 days from the date of acceptance.
XI. Authority of Implementation:
Implemented by: CFDA
Accepted at: CFDA Administrative Service Center
XII. Changes:
For alterations of registration and licensing items after the approval of IVD registration, the applicants shall apply for alterations to relevant drug administration departments. For alterations of registration items, the applicant shall apply for the changes within 30 days from the date of the change; for alterations of licensing items, the applicant shall apply for the changes and implement them after approval, if alterations of licensing items occur within six months before the expiry of the registration certificate, application for change can be made along with renewal of registration. If marketed products changed the basic reaction principle or analytical sensitivity index with a new clinical diagnostic significance, they shall be handled in accordance with the initial application for registration.
XIII. License validity and renewal:
Medical device registration certificate is valid for four years. The applicant shall apply for the renewal of registration to relevant drug administration departments within six months before the expiry of the registration certificate. If the production and sales of a product are still necessary after renewal of registration before the expiry of the registration certificate, the applicant shall apply in accordance with the procedures and requirements for initial registration.
XIV. Annual inspection or annual review of the license: None
XV. Institutions for inquiries and complaints:
Inquiries: CFDA
Complaints: CFDA Bureau of Investigation and Enforcement, Department of Legal Affairs
Note: The time frame of this Notice counts on working days, excluding legal holidays

