| 3 Submission Requirements of the Standard DataThe following materials are needed for the third kind of scale:2 Product instruction;4 Analysis of the data for performance ev |
Instructions to Registration, Submission and Test of in Vitro Diagnostic Reagents Beijing Institute of Medical Device Testing
1. Scope of Application
The products that need registration and test which are defined as in vitro diagnostic reagents and belong to medical device regulation by in Vitro Diagnostic Reagents Registration Regulation Rules (Tentative Rules).
2. Submission and Test Flow Chart
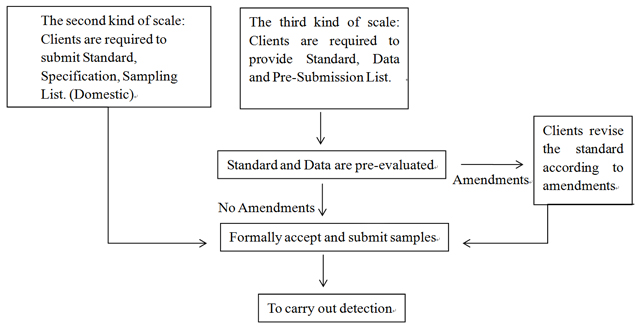 3. Submission Requirements of the Standard Data
3. Submission Requirements of the Standard DataThe following materials are needed. Stamping with the manufacturing enterprise seals. Importers can stamp with the China Office seal. The third kind of scale must concurrently provide the statement to guarantee the authenticity of data.
The following materials are needed for the third kind of scale:
1.Sampling list (sample certification and record);
2.Product instruction;
3.Product standard and product preparation instructions;
4.Analysis of the data for performance evaluation;
Following contents should be included at least:
a)Test items: analyzing sensitivity and specificity, test scope, detection accuracy, imprecision of the same batches, imprecision between different batches, etc. ;
b)Test methods: type, trademark, and name of the experiment instrument, calibrating sample (the standard strain), samples for quality control or other use; detailed experimental protocols and computing methods.
c) Data and results.
5. Data of stability research: it should at least include: date, items, methods and results.
6.Package and label.
7.Standard sample and quality control;
a)When applying for reagents�� registration and test, you should provide brief evaluation if the standard sample and control sample are produced by yourself; if not, you ought to provide trademark and type clearly.
b)When applying for the registration and testing of the calibrating sample and control sample, information and data must be provided according to Requirements of the Calibrating Original Document and Control Sample Evaluating Procedure Document.
4. Requirements of samples needed to be submitted and test
1.Requirements of quantity
Generally speaking, quantity of samples should be three times of testing amount per time. When confronted to special circumstances (e.g. valuable samples, special controlled in vitro diagnostic reagents, etc.), you should provide a written explanation. However, the testing samples shouldn��t be less than testing and re-examining amount. In the meantime, you must indicate ��Do Not Apply for Re-examination�� in the application letter or the testing application form and make a signature.
2.Requirements of batch
a) Requirements of batch for the third kind of scale are: three consecutive batches within the validity period and one batch beyond the validity period (providing the product beyond the validity period according to standard). A statement is needed for imported products to prove the authenticity of three consecutive bathes presented by manufacturers.
b) Requirements of batch for the second kind of scale are: three bathes with the validity period and one batch beyond the validity period (control samples and calibrating samples are the product of one batch within the validity period).
3.Requirements of sample state
a)In vitro domestic diagnostic reagents should be sampled drawn by the local Food and Drug Administration. The seals should be intact, and signatures and seals are clear.
b)The imported in vitro diagnostic reagents extracted by the applicant, and testing samples should be the finished product (contents should be consistent with the specification). Package is intact, damage-free, and the logo or label is clear.
c)Submission samples should have an intact package, and one testing amount at least.
4.Requirements of auxiliary sample
a)Providing samples of control, calibrators, and the linear high-value within the period conforming to the standard. And the quantity should be able to meet the needs of all trials.
b)If the testing needs special apparatus, the special apparatus should be provided by the enterprise, and the metrology / calibration certificate also should be provided.
5. Formally acceptance Notes
After the end of the standard data pre-assessment process, the next step is the formally acceptance.
1.Fill in the form Power of Attorney for Medical Device Product Testing, Registration Card, Notification of the Task (triple)
Applicants should carefully fill out the power of attorney (triple). Samples�� name should first comply with the provisions of the In Vitro Diagnostic Reagents Registration (Trial). And the name on packaging should be consistent with the registered standard samples and specification.
2.Submitting the registered product standard
Applicants should explain whether the standard is modified in accordance with the standard of our center.
3.Submitting and testing samples
Submitting and testing samples should be done according to the requirements of Notice of the Standard Opinion of In Vitro Diagnostic Reagents and Supplementary Sample putting forward by our center after the pre-evaluation according to the standard.
(From Beijing Institute Of Medical Device Testing)

