| Charging Standard for Registration Fees of Drug and Medical Device Product1 Drug registration feesThe food and drug supervision and administration department under managemen |
Attachment 1
Charging Standard for Registration Fees of Drug and Medical Device Product
1. Drug registration fees
The food and drug supervision and administration department under management of the State Council and the provincial food and drug supervision and administration departments carry out the administrative processing, on-site inspection/verification, technical review and other registration work for clinical trials and production applications of new drugs, generic drug application, supplementary application and re-registration in accordance with legal responsibility and charge fees according to relevant standards. The specific charge standards are as follows:
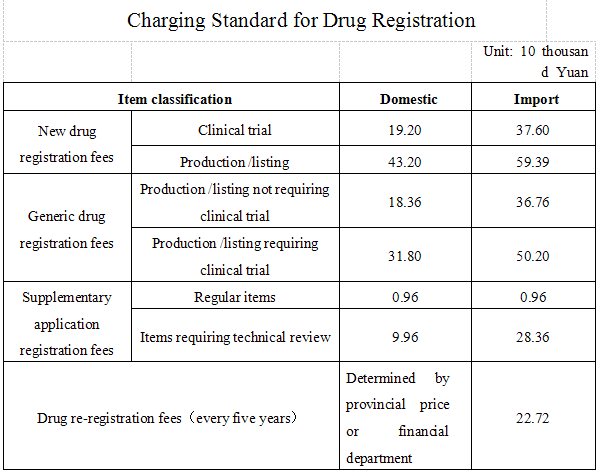
Note:
1.For drug registration fees, the fees are counted by considering an API or a preparation as a variety, if adding a specification, then 20% additional registration fee will be added on the basis of the corresponding category.
2. For the supplemental application matters under management of provincial food and drug supervision and administration departments or direct record to food and drug supervision and management departments under the state council, no supplement registration fees will be charged, technical review is required for such kind of application after review, then the applicant shall pay the fees in accordance with the charging standard for technical review of supplement application.
3. For application of disposable imported drugs, 2000 Yuan drug registration fee will be charged.
4. The registration fee standards for imported drug is calculated by counting the difference of domestic and abroad transportation, accommodation and meals in addition to relevant registration fees for domestic drugs.
5. The imported drug registration fee standards are applicable to the registration fee standards for drugs from Hong Kong, Macao and Taiwan drug.
6. Standard for surcharge of drug registration shall be separately determined.
2. Medical device registration fees
The food and drug supervision and administration department under management of the State Council and the provincial food and drug supervision and administration departments carry out administrative processing, quality management system verification, technical review and other registration work for initial application, alteration registration, continuation registration of Class II and Class III medical device as well as clinical trials of Class III high-risk medical device in accordance with legal responsibility and charge fees according to relevant standards. The specific charge standards are as follows:
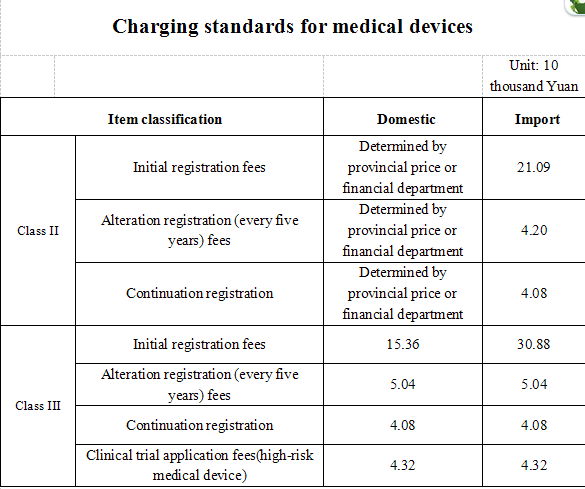
Note:
1. The registration fee charging for medical device products according to the registration units as determined in Measures for Registration of Medical Device and Measures for Registration of In Vitro Diagnostic Reagents.
2. No change registration fees will be charged for the change of those record matters for recording as specified in the Measures for Registration of Medical Device and Measures for Registration of In Vitro Diagnostic Reagents.
4. The registration fee standards for imported drug is calculated by counting the difference of domestic and abroad transportation, accommodation and meals in addition to relevant registration fees for domestic drugs.
5. The imported drug registration fee standards are applicable to the registration fee standards for drugs from Hong Kong, Macao and Taiwan drug.
6. Standard for surcharge of medical device registration shall be separately determined.

